THE CHEMISTRY OF CHOLESTEROL AND ALCOHOL: Oxidation of Alcohols
As a working definition in organic chemistry, oxidation can be considered as either the gain of an oxygen atom by a substance or the loss of two hydrogen atoms from it. Since reduction is the chemical opposite of oxidation, reduction can be considered as either the gain of two hydrogen atoms or the loss of one oxygen atom. These atoms are always denoted by [O] and 2[H]. The square brackets signify that we are not referring to atomic hydrogen or oxygen. The substance that supplies the oxygen, [O], and/or removes the hydrogen, 2[H], is called the oxidizing agent or oxidant. The reducing agent or reductant removes the oxygen, [O], and/or supplies the hydrogen, 2[H].
OXIDATION OF PRIMARY ALCOHOLS INTO ALDEHYDES
Considering butan-1-ol. The removal of two hydrogen atoms produces a new functional group – the aldehyde group, CHO. In the oxidation of a primary alcohol, the reagent is acidified aqueous potassium dichromate(VI), a mixture of aqueous potassium dichromate(VI) and dilute sulphuric acid. The conditions must be chosen to prevent any subsequent oxidation of the aldehyde to form a carboxylic acid. Either the reaction is carried out at room temperature and the oxidizing agent is added to the alcohol so that the oxidizing agent is never in excess, or the reagents are heated and the aldehyde product is allowed to distil out from the reaction vessel before it can be oxidized further.
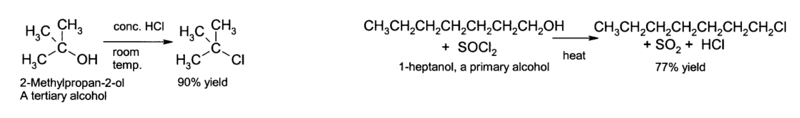
During the oxidation, orange dichromate(VI) ions are reduced to form blue-green chromium(III) ions. In this equation, the oxidizing agent is not given in full, but is represented by the oxygen [O] that it supplies to remove the two hydrogen atoms. This is a much simpler expression of the reaction than the full stoichiometric or ionic equation for the reaction. The oxidation of ethanol by acidified potassium dichromate(VI) to give ethanal can be written as:
CH3CH2OH + [O] → CH3CHO + H2O
Stoichiometric and half equations
The stoichiometric equation for the oxidation of a primary alcohol is very complicated and tends to hide the processes taking place. For example, in oxidation by potassium dichromate(VI):
3RCH2OH + K2Cr2O7 + 4H2SO4 → 3RCHO + K2SO4 + Cr2(SO4)3 + 7H2O
Even the slightly simpler ionic equation is still complicated:
3RCH2OH + Cr2O72- + 8H+ → 3RCHO + 2Cr3+ + 7H2O
It is easier to look at the oxidation half equation for the reaction. During the reaction, the alcohol loses two electrons, as in the half equation:
RCH2OH → RCHO + 2H+ + 2e‑
The electrons supplied by the oxidation of the alcohol reduce the dichromate(VI) ion.
Cr2O72- + 14H+ + 6e‑→ 2Cr3+ + 7H2O
OXIDATION OF PRIMARY ALCOHOLS INTO CARBOXYLIC ACIDS
By changing the conditions of oxidation, it is possible to remove two hydrogen atoms and gain one oxygen atom to produce a carboxylic acid. The oxidation is represented by:
RCH2O + 2[O] → RCO2H + H2O
The alcohol is refluxed with acidified potassium dichromate(VI). The acid used is dilute sulphuric acid. Ethanol is oxidized to give ethanoic acid by refluxing with acidified potassium dichromate(VI). The reaction can be represented as:
CH3CH2OH + 2[O] → CH3COOH + H2O
The half equation for the oxidation of ethanol into the carboxylic acid involves the loss of four electrons from ethanol. This corresponds to the gain of [O] and the loss of 2[H]:
H2O + CH3CH2OH → CH3COOH + 4H+ + 4e-
Ethanol can sometimes be oxidized by atmospheric oxygen. This can happen during fermentation, giving the ethanol a vinegary taste:
CH3CH2OH + O2 → CH3COOH + H2O
OXIDATION OF SECONDARY ALCOHOLS INTO KETONES
Secondary alcohols can be oxidized to give ketones rather than aldehydes.
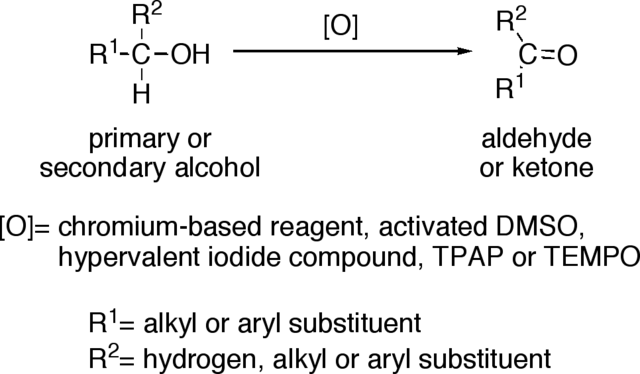
Oxidation of alcohols to aldehydes and ketones. Gabriel Tojo , CC BY 2.5
The alcohol is refluxed with the acidified potassium dichromate(VI). Sulphuric acid is used to acidify the reagent. The reaction involves the removal of two hydrogen atoms. So, for example, propan-2-ol is oxidized to give propanone as follows:
3CH3CHOHCH3 + Cr2O72- + 8H+ → 3CH3COCH3 + 2Cr3+ + 7H2O
Notice that I have used the ionic formulae and left out the potassium ions, which remain unchanged during the reaction. The half equation for the oxidation of a secondary alcohol involves two electrons and is similar to the half equation for a primary alcohol being oxidized to give an aldehyde. Another simpler way to represent the oxidation of propan-2-ol is:
CH3CHOHCH3 + [O] → CH3COCH3 + H2O
Cutting costs to make propanone
Propanone is an important industrial and domestic solvent. It is manufactured from propan-2-ol, but acidified potassium dichromate(VI) is not used because the reagents are expensive and the special conditions required are difficult to maintain in a large industrial plant. Instead, propan-2-ol vapour is passed over a copper catalyst at 300 °C, which removes hydrogen molecules in a reaction called dehydrogenation.
OXIDATION OF TERTIARY ALCOHOLS
It is impossible to oxidize a tertiary alcohol without breaking a carbon-carbon bond. Acidified potassium dichromate(VI) has no effect on tertiary alcohols.
DISTINGUISHING PRIMARY, SECONDARY AND TERTIARY ALCOHOLS
Since the three classes of alcohols give different reactions with acidified potassium dichromate(VI) they can be distinguished from one another by the products and colour changes in these reactions, as the table below shows.
A table distinguishing alcohols
| Alcohol | Acidified potassium dichromate (VI) at room temperature | Refluxing under heating with acidified potassium dichromate (VI) | ||
| Product | Observation | Product | Observation | |
| primary | aldehyde | orange to green | carboxylic acid | orange to green |
| secondary | ketone | orange to green | ketone | orange to green |
| tertiary | no reaction | stays orange | no reaction | stays orange |
COMBUSTION OF ALCOHOLS
Simple alcohols such as methanol or ethanol will combust completely to form carbon dioxide and water. The presence of the oxygen atom in the molecule of methanol or ethanol means that less oxygen is needed for complete combustion than with the corresponding alkanes.
C2H5OH + 3O2 → 2CO2 + 3H2O
Alcohols with many carbon atoms per molecule are much more likely to burn incompletely in air producing a dirty flame and lots of soot. Ethanol is a possible biofuel because it can be made by fermentation of sugars from plants. The combustion products, carbon dioxide and water, can be incorporated into plant carbohydrate by photosynthesis. It is therefore possible for ethanol to be a carbon-neutral biofuel, having no net effect on carbon dioxide concentrations in the air.
TRI-IODOMETHANE TEST FOR CH3CHOH GROUP
Alkaline aqueous iodine reacts with ethanol and secondary alcohols containing the CH3CHOH group to give a yellow precipitate of tri-iodomethane. This reaction is used as a chemical test in analysis and identification.
ALCOHOLS AS ACIDS
RELATIVE ACIDITY OF ALCOHOLS
An acid is a proton donor. An alcohol ca behave as an acid because it can donate a proton if the O – H bond is broken. So, the weaker the O – H bond is, the stronger is the acidity of the alcohol. The strength of the O – H bond can be changed by changing the atoms attached to it. For example, electron-releasing groups strengthen the O – H bond and thereby make the alcohol a weaker acid.
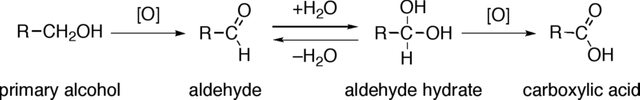
Oxidation of primary alcohols to carboxylic acids. Gabriel Tojo, CC BY 2.5
Alkyl groups are electron releasing compared with the hydrogen atom, so it follows that the order of increasing acid strength in alcohols is: tertiary, secondary, primary (strongest). Water, which also contains the O – H bond, is a stronger acid than primary alcohols.
REACTION OF SODIUM WITH ALCOHOLS
Sodium reacts violently with water to form hydrogen and aqueous sodium hydroxide. In the reaction, an O – H bond is broken:
2H2O(l) + 2Na(s) → 2NaOH(aq) + H2(g)
Ethanol reacts similarly with sodium, to produce hydrogen and a compound called sodium ethoxide. In the reaction, the sodium fizzes and produces a colourless solution. The reaction is slower than that of sodium with water, because the O – H bond in ethanol is harder to break than the O – H bond in water
2C2H5OH + 2Na → 2C2H5O-Na+ + H2
Notice that the two equations show the same pattern. Sodium reacts with other alcohols, to produce hydrogen and what is known as an alkoxide. The name of the alkoxide is derived from the alkyl chain of the alcohol:
2ROH + 2Na → 2RO- Na+ + H2
The alcohol in this example acts as a very weak acid, since it loses a proton RO-Na+ is known as a sodium alkoxide.
SUMMARY
After going through all my previous articles on the chemistry of cholesterol and alcohols #1, #2, you should know that:
- Alcohols contain the hydroxyl, OH, functional group attached directly to a carbon atom.
- Short-chain alcohols are soluble in water, but long-chain alcohols are insoluble.
- Primary alcohols can be oxidized by acidified potassium dichromate(VI) to give aldehydes. The aldehyde is often distilled from the reaction mixture as it is being made to prevent further oxidation.
- Primary alcohols can be oxidized by hot acidified potassium dichromate(VI) to give carboxylic acids.
- Secondary alcohols can be oxidized to give ketones, but tertiary alcohols cannot be oxidized.
- Alcohols completely combust to form carbon dioxide and water.
- Alcohols can be converted by substitution into halogenoalkanes, using phosphorus halides and the appropriate hydrohalic acid, HBr(aq), HCl(aq) or HI(aq)
- Alcohols can be dehydrated to give alkenes.
- Alcohols can behave as nucleophiles and react with carboxylic acids and acid chlorides to form esters.
- Many alcohols are used as solvents.
- Ethanol is manufactured by the anaerobic fermentation of aqueous glucose or by the hydration of ethene in the presence of an acid catalyst.
- Alcohols react with sodium to give sodium alkoxide.
Thanks for reading.
REFERENCES
Oxidation of primary alcohols.
https://en.wikipedia.org/wiki/Oxidation_of_primary_alcohols_to_carboxylic_acids
https://www.organic-chemistry.org/synthesis/C2O/ketones/oxidationsalcohols.shtm
https://en.wikipedia.org/wiki/Oxidation_of_secondary_alcohols_to_ketones
https://en.wikipedia.org/wiki/Alcohol_oxidation
https://en.wikipedia.org/wiki/Lucas%27_reagent
https://www.chemguide.co.uk/organicprops/alcohols/oxidation.html
https://pubs.acs.org/doi/abs/10.1021/ed030p395
http://www.passmyexams.co.uk/GCSE/chemistry/combustion-of-alcohols.html
https://www.ausetute.com.au/combustol.html
https://www.chemguide.co.uk/organicprops/alcohols/iodoform.html
https://www.chemguide.co.uk/organicprops/carbonyls/iodoform.html
https://www.britannica.com/science/alcohol/Reactions-of-alcohols

Hello,
Your post has been manually curated by a @stem.steem curator.
We are dedicated to supporting great content, like yours on the STEMGeeks tribe.
Please join us on discord.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.