The Chemistry of Cholesterol and Alcohol.
Cholesterol is a member of the class of
chemicals called steroids, which are both naturally occurring and synthetic.
The most abundant steroid is cholesterol, which (as the ‘ol’ at the end of its
name suggests) contains an alcohol group. It is found in practically all animal
tissues, and forms an important part of cell membranes. Cholesterol is
particularly abundant in the brain and spinal cord. It is estimated that the
total amount of cholesterol in 75 kg person is about 250 g.
High levels of cholesterol in the blood are associated with two medical conditions, gallstones and atherosclerosis (hardening and thickening of the arteries when cholesterol from the blood builds up on their inside walls). Blood cholesterol levels can now be measured easily, and people with a high level are advised to reduce their intake of cholesterol-rich foods and saturated fats.
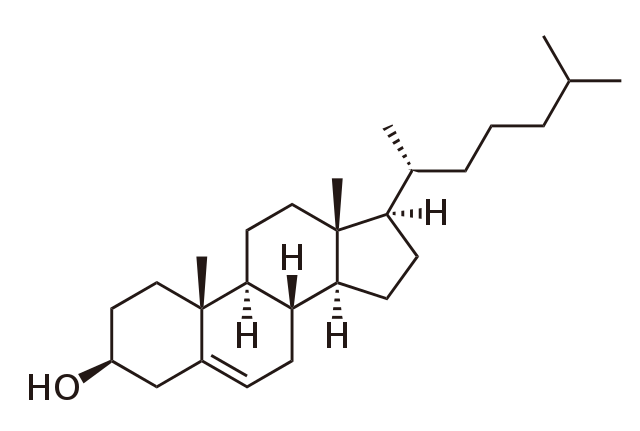
Chemical structure of cholesterol. BorisTM, Public Domain
Cholesterol was first isolated in 1770, but it took almost another two centuries before the full structure was established. As the diagram shows, cholesterol has a complicated structure that contains four rings and two functional groups.
THE HYDROXYL GROUP
Alcohols are organic compounds that contain carbon, hydrogen and oxygen. They contain the hydroxyl functional group, OH, which is an oxygen atom bonded to a hydrogen atom. The hydroxyl group is bonded directly to an alkyl or cycloalkyl group.
There is sometimes confusion in the use of the name alcohol, because it is the name of a class of compounds as well as the name of a particular compound. Alcohol is the popular term for ethanol. But in this post, you can safely assume that when we refer to alcohols we mean the class of compounds – not the single compound.
ALCOHOLS IN NATURE
Many naturally occurring products are alcohols, including sugars and other carbohydrates, some fragrances, vitamins, pheromones, amino acids and steroids. Serine is an amino acid used by organisms in the construction of proteins. The structure of serine is shown below:
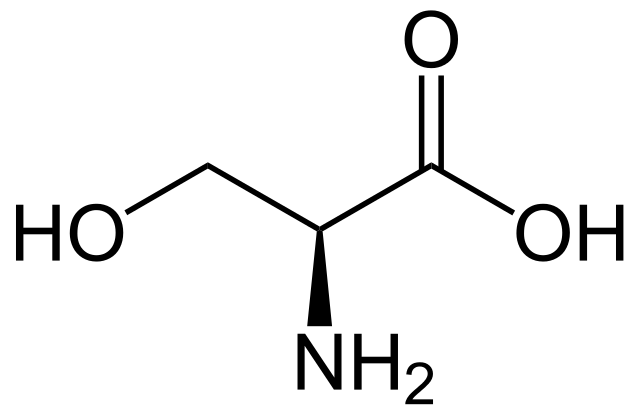
Structure of L-serine. NEUROtiker, Public Domain
The structure of serine, an amino acid that is also an alcohol
A lack of the alcohol vitamin A in a person’s diet causes night blindness and dry skin. And without sufficient vitamin C – another compound that contains alcohol functional groups – a person would suffer from scurvy.
Phenols are compounds that contain a hydroxyl
group directly attached to a benzene ring. You can read more about phenols on
one of my previous post on AROMATIC HYDROCARBONS. In order to be able to
recognize an alcohol when it is part of a very complicated molecule, just look
for the hydroxyl group attached to a carbon atom.
Vitamin A is a polyene and also a primary alcohol while Vitamin C is a cyclic molecule with more than one alcohol group
The fragrance of scented flowers is the result of a complex mixture of compounds, many of which are alcohols. Propane-1,2,3-triol is used to make fats and oils that the human body uses as energy stores and insulation, and also in the construction of membranes that surround living cells. Polysaccharides contain many hydroxyl groups.
ALCOHOLS IN USE
Alcohols with low relative molecular mass, such as methanol, ethanol, the propanols and the butanols, are liquids and are used extensively as solvents, both for chemical reactions and in the production of paints and cosmetics. Many alcohols have the ability to dissolve both polar and non-polar compounds. This property is explained later in terms of the different types of inter-molecular attraction between alcohol molecules and other molecules. Alcohols are also very useful intermediates in the synthesis of compounds, since they have a functional group that can undergo different types of reaction.
NAMING ALCOHOLS
The name of an alcohol is derived from the name of its carbon chain, using the suffix ol. The position number for the hydroxyl group must also be specified in the name. The rule is that the carbon atom attached to the hydroxyl group is given the lowest number possible. When there is more than one functional group present, the position numbers are determined by the most important group for naming purposes. (For example, the position of the hydroxyl group is more important than the position of a double bond or of halogen atom, but less important than the position of a carbonyl group.) The presence of two or more hydroxyl groups is designated by di, tri, and so on, placed immediately before the suffix ol.
TYPES OF ALCOHOL
There are two ways of classifying alcohols. One is based on the number of carbon atoms attached to the carbon to which the hydroxyl group, OH, is bonded. The other is based on the number of hydroxyl groups present per molecule.
PRIMARY, SECONDARY AND TERTIARY ALCOHOLS
A primary alcohol has one carbon attached to the carbon to which the OH group is bonded, a secondary alcohol has two carbon atoms attached and a tertiary alcohol has three carbon atoms attached. Although all alcohols have some reactions in common, their other reactions are different and depend on whether they are primary, secondary or tertiary alcohols.
For convenience, methanol is classified as a primary alcohol, even though it does not have a carbon atom bonded to the carbon atom to which the OH group is attached.
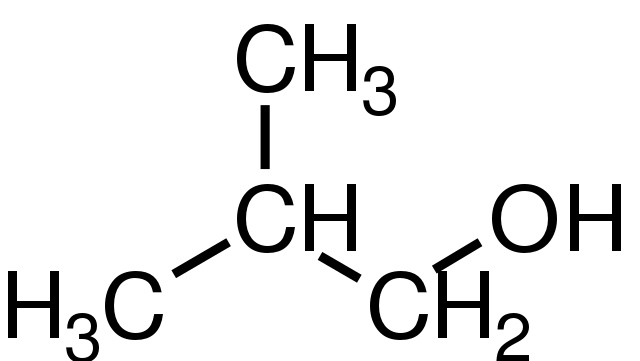
2-methylpropan-1-ol. GKFX, Public Domain
Diagram of secondary alcohol
MONOHYDRIC, DIHYDRIC, TRIHYDRIC AND POLYHYDRIC ALCOHOLS
A monohydric alcohol has one hydroxyl group, a dihydric alcohol has two, trihydric alcohol has three and a polyhydric alcohol has many hydroxyl groups per molecule. Ethane-1,2-diol is a dihydric alcohol used in engine antifreeze. Glucose is polyhydric alcohol with many hydroxyl groups per molecule
ALCOHOLS AS A HOMOLOGOUS SERIES
Methanol, ethanol, propan-1-ol, butan-1-ol and pentan-1-ol are alcohols that are members of a homologous series. They are all primary alcohols with similar chemical properties, and can be represented by the general formula CnH2n+1 OH. As in all homologous series, the physical properties of each member show observable trends as the number of carbon atoms per molecule increases.
A table showing the physical properties of the homologous series of primary alcohols
| Alcohol | Formula | Melting point /°C | Boiling point/°C |
| methanol | CH3OH | -98 | 65 |
| ethanol | CH3CH2OH | -117 | 78.5 |
| propan-1-ol | CH3CH2CH2OH | -126 | 97 |
| butan-1-ol | CH3CH2CH2CH2OH | -90 | 116 |
| decan-1-ol | CH3(CH2)8CH2OH | 6 | 228 |
BOILING POINTS
The table above shows that the boiling points of the primary alcohols CnH2n+1 OH increase with the carbon chain length, n. For an alcohol to boil, its molecules must overcome the two types of inter-molecular force that keep them together. One is the weak induced dipole-induced dipole force that operates between all molecules as a result of the temporary dipoles within the molecules. This force becomes stronger as the molecule becomes larger and contains more electrons. The induced dipole-induced dipole force in alcohols is the attraction between the alkyl part of one molecule and the alkyl part of another molecule.
The other inter-molecular force is the much stronger hydrogen bond. The hydroxyl group has a polar covalent bond in which the oxygen atom is slightly negative (ƍ-) and the hydrogen atom is slightly positive (ƍ+). This is because the oxygen is much more electronegative than hydrogen. This results in the molecule having a permanent dipole. The negative oxygen atom attracts the positive hydrogen atom of the hydroxyl group of another molecule, forming the hydrogen bond.
SOLUBILITY IN WATER
The solubility of the primary alcohols CnH2n+1 OH decreases as the number of carbon atoms per molecule increases. Methanol and ethanol mix completely with water in all proportions, whereas nonan-1-ol, in which n = 9, is virtually insoluble in water. For a molecule to dissolve in water, it must be able to interact with water molecules. Water is a polar solvent, so the two hydroxyl groups in a water molecule can form inter-molecular hydrogen bonds with methanol or ethanol molecules.
In the case of nonan-1-ol, its hydroxyl groups form hydrogen bonds with the water molecules, but its long carbon chains form induced dipole-induced dipole interactions only with other nonan-1-ol molecules. Therefore. The predominant intermolecular attractions are between nonan-1 ol’s molceules rather than between nonan-1-ol and water molecules.
SUGARS
Sugars are a group of naturally occurring polyhydric alcohols that normally have a carbon chain or ring of six or five carbon atoms. They also contain ketone group or one aldehyde group.
Glucose exists in both a chain form and a ring form. Both forms have a molecule with five hydroxyl groups. It readily dissolves in water because its hydroxyl groups can form hydrogen bonds with water molecules. It is this solubility that enables us to taste sugar in food or drink.

ARTIFICIAL SWEETENERS
Diabetics do not produce sufficient insulin to change the excess glucose in their blood into glycogen, a storage carbohydrate. So they must carefully regulate their intake of glucose to stop the blood sugar level from getting too high. But like other people, diabetics like sweet-tasting foods. The solution is to use artificial sweeteners, such as sorbitol, which is used to sweeten diabetic chocolate and other confectionery. The 3-D shape of the sorbitol molecule is very like that of the glucose molecule. The receptors in the tongue respond to sorbitol in the same way that they respond to glucose, and so sorbitol tastes sweet. However, despite sorbitol’s similar taste to glucose, it is not metabolized by the body, and a diet too rich in sorbitol can cause diarrhoea.
Sorbitol is also used to sweeten dry wines. Adding extra sugar would sweeten the wine, but would also promote unwanted additional fermentation with the production of carbon dioxide, and the pressure could burst the bottle. Since sorbitol is not metabolized by yeast, further fermentation is not a problem. The market for artificial sweeteners goes beyond helping diabetics and wine-making. Many low-calorie products have now replaced sugar as an ingredient. However, many artificial sweeteners chemically decompose with heating, so they cannot be used to sweeten products that are cooked, such as cake mixtures.
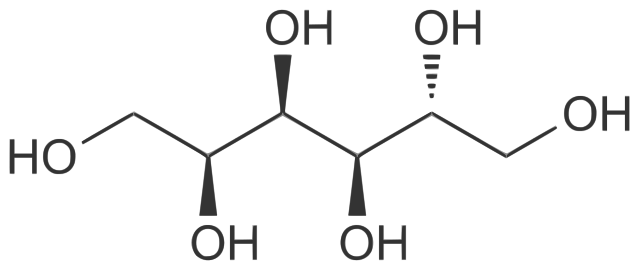
Chemical structure of D-sorbitol. Mrgreen71, CC BY-SA 3.0
Most artificial sweeteners are much sweeter than sugar, so a much smaller mass of the substance will be needed. Saccharin, for example, is about 300 times sweeter than sugar, though it has a bitter aftertaste. Another sweetener is aspartame, which is 200 times sweeter than sugar, but some people experience unpleasant effects from it, such as headaches and nausea. Molecules of artificial sweeteners have a different shape from those of the simple sugars, so chemists are not yet sure which molecular shapes are responsible for the sweetness. Recent research has attempted to make sugar-like molecules by substituting some OH groups with chlorine atoms. But there must be extensive testing before such products can be put into foods, in order to ensure that they are completely free of side effects.
Thanks for coming.
REFERENCES
https://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/symptoms-causes/syc-20350800
https://medlineplus.gov/cholesterol.html
https://www.masterorganicchemistry.com/2010/06/16/1-2-3-4/
https://byjus.com/chemistry/types-of-alcohols/
https://www.youtube.com/watch?v=_mdoeSMiPxU
https://brainly.in/question/8806486
https://en.wikipedia.org/wiki/Homologous_series
https://www.bbc.co.uk/bitesize/guides/z8sb2p3/revision/1
https://foodinsight.org/background-on-carbohydrates-sugars/
https://en.wikipedia.org/wiki/List_of_sugars
https://en.wikipedia.org/wiki/Sugar
https://en.wikipedia.org/wiki/Sugar_substitute
https://www.health.harvard.edu/blog/artificial-sweeteners-sugar-free-but-at-what-cost-201207165030
https://en.wikipedia.org/wiki/Sorbitol
Hello there @empressteemah :)
Wonderful post! I liked chemistry quite a lot when I studied it back in the high school days. But I (sadly) remember very little about compounds classes and properties. So, I just wanted to let you know I thoroughly enjoyed reading this piece. Particularly interesting to me was your brief discussion about sorbitol. Especially the part you explained its use in wine-making.
Thank you for providing us with this straight-forward, informative read my dear.
I wish you a wonderful day ahead! :D
All the best,
Abigail
Thanks, dear @abigail-dantes.
I really love your comments. Thanks a bunch.
I wish you a great weekend ahead!
Love you, dear.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Good profile and post
Posted using Partiko iOS
Thanks for coming, @stefano.massari
Your post has been curated by the bitcoin myk project. Tokens are available for this account you can trade for steem at: https://steem-engine.com/. Join our curation priority list to earn more tokens by registering at:
http://www.bitcoinmyk.com/register/
Bitcoin MYK
admin
Register - Bitcoin MYK
Thanks, @bearbear613