Reactions with Potassium Permanganate. The Chemical Chameleon
Greetings friends.
We can find chemical reactions of different types, and some surprise us by the color changes that they produce in the solutions. Unexpectedly in this, the reactions with potassium permanganate make it a chameleon of chemistry. Due to this versatile property of exhibiting different colors associated with different oxidation states, it is widely used to perform demonstrations in the classroom of redox reactions.

The colors of potassium permanganate, the chemical chameleon. Own image.
Potassium permanganate
This chemical compound has a characteristic purple color, it is a salt composed of potassium ions (k+) and permanganate (MnO4-), in this the manganese is using its oxidation state +7 with an electronic structure 3s2,3p6,3d0, this vacancy in d make it a strong oxidant, which is why it is widely used in redox (reduction-oxidation) reactions.
The permanganate ion forms a tetrahedral structure that, unlike other ions with the same structure as perchlorate (ClO4-), does not produce colorless solutions. Instead, electronic bands are produced in the solid capable of absorbing energy in the range of 18,000 cm-1, filtering the green color and exhibiting that characteristic purple color that remains in aqueous solutions.
Oxidation number and Redox reactions
As we know, in the reactions of oxidation and reduction an exchange of electrons between the reactants takes place, which produces a change in the number of oxidation of the reactive species. This oxidation number is the positive or negative electric charge, which is assigned to an atom or ion. The reduction and oxidation reactions (also called redox) are constituted by two types of reagents, one called oxidizing agent and the other reducing agent. In the reaction, the oxidizing agent gains electrons and reduces its oxidation number (this species undergoes the reduction), while the reducing agent loses electrons and increases its oxidation number (it is the species that is oxidized). Both processes occur simultaneously.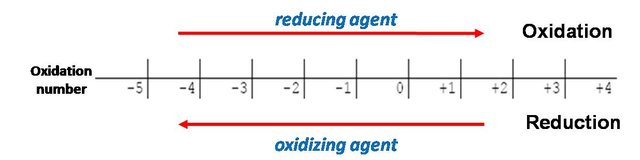
Oxidation-reduction scale. Own image.
Let's see some colorful examples that involve potassium permanganate.
Basic solution reaction
If an alkaline solution, such as the one used in the following example, which was prepared by dissolving 2 mL of 6N sodium hydroxide in 60 mL of water, added 0.01M potassium permanganate, we obtain the following reaction.

This reaction is kind of slow, but it can be better appreciated if we raise the temperature to about 80 ºC. It can be seen what happens in the next video.
Reaction of potassium permanganate in basic medium. Video made by me.
The video shows the reduction of green permanganate to KMnO42-. The process can be bettervisualized if we build semi-reactions,
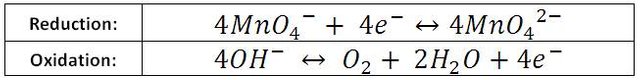
In the first one, the gain of electrons is shown, therefore it is the reduction reaction. Except for a few cases, oxygen always uses the number of oxidation -2 in its compounds, so the manganese of the permanganate ion goes from +7, its highest state of oxidation, to +6 forming the ion manganate (MnO42-). As illustrated in the following semi-reaction:
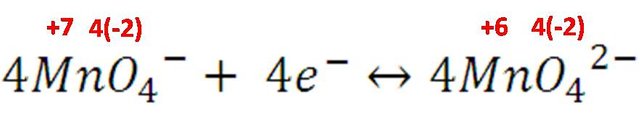
As it was mentioned before, the tetrahedral structure of MnO42- produces intense colors, in this case the electronic transition produces bonds capable of absorbing the spectrum of the purple color, producing green color instead.
By adding the permanganate solution it can be seen for a moment a blue color, this can be produced by the formation of the ion hipomanganate (MnO43-), however this complex is very unstable at high pH, so it disappears almost instantaneously.
Reaction with hydrogen peroxide in acid medium
If wemake react potassium permanganate with hydrogen peroxide, in an acidified solution with a few drops of sulfuric acid, we will observe the reduction of permanganate to manganese dioxide (MnO2), which is a brow solid. The process is described by the following reaction:
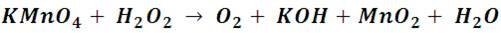
The reduction semi-reaction shows that manganese is reduced from +7 to +4.
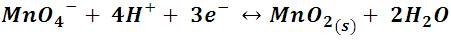
Reaction of potassium permanganate with hydrogen peroxide. Video made by me.
Reaction with sucrose in basic medium
When we do reacting sucrose (sugar) with potassium permanganate in a basic medium, we get an eye-catching reaction known as the chemical chameleon. What happens is that during the reaction takes place the oxidation of sucrose and a gradual reduction of potassium permanganate, from its oxidation state +7 in the permanganate ion, through its oxidation state +6, up to +4 ,we will be able to appreciate this by means of the changes of color previously observed, from purple to green, from green to yellow-brown, only that now forming part of the same reaction.
How can we do it:
1.Materials:
- An erlenmeyer.
- A hot-plate with magnetic stirring.
- 3 g of sugar.
- 3 mL of 6N NaOH.
- Distilled water.
2. Procedure:
- Add 200 mL of distilled water to the Erlenmeyer flask and pour 3 g of sugar and 3 mL of NaOH. Stir until dissolved.
- We prepare a 0.01 M solution of KMnO4.
- Place the Erlenmeyer flask on the hot plate and activate the stirring function.
- Slowly add 3 mL of the KMnO4 solution.
- And we observed the incredible result, as in the next video.
Reaction known as chemical chamaleon. Video made by me.
The different colors are due to the different oxidation states of manganese. In sucrose there are groups of alcohols that are oxidized in alkaline solutions producing ketones, and in the basic medium with excess sugar the permanganate is reduced to manganate (green) as explained above. In the next stage, the manganate is reduced by forming the manganese dioxide by the following reaction:
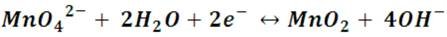
At low concentrations MnO2 does not precipitate, so the solution instead of turning brown with a dark brown solid dispersed in the medium (as in the reaction with oxygenated water), then it remains soluble and the solution acquires that color yellow toasted.
As can you see, using basic knowledge of redox reactions can generate an entertaining and didactic experience, enjoying, as in this case, the striking colors that give us the reactions with potassium permanganate. Until next time!
Thanks for reading.
References
Chang, R. (2006). Chemistry. McGraw-Hill.
Skoog, West, Holler, Crouch. (2001). Analitic chemistry.
Wikipedia.com. Chemical chamaleon.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider setting @steemstem as a beneficiary to your post to get a stronger support.
Thanks for having used the steemstem.io app. You got a stronger support!
thank you very much friends!!
Hi @emiliomoron!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Thanks for the support!