Carbon Monoxide Poisoning - The Chemistry
Low-level exposure to carbon monoxide gas causes symptoms similar to those of the flu. High-level exposure, on the other hand, rapidly leads to intoxication, ataxia (complete loss of coordination), tachycardia (dangerously high heart rate) and seizures. Loss of consciousness and death follows in a matter of minutes.
In the UK, the installation of carbon monoxide detectors in addition to standard fire alarms has been mandatory for all landlords and homeowners since 2015. The change in legislation followed a spate of deaths (53, according to the Office for National Statistics https://goo.gl/sruhjE) and has since dramatically reduced the number of reported fatalities.
But what is carbon monoxide and why is it so dangerous?
Carbon Monoxide Sources
Carbon monoxide is a molecule comprising one carbon atom and one oxygen atom, and exists as a gas at room temperature and is completely odourless - hence its moniker, 'the silent killer' - making high-level exposure very difficult to diagnose in time. It is the product of incomplete combustion - the burning of carbon-based fuels (such as wood and gas) in insufficient oxygen for the production of carbon dioxide alone - and can result from a number of everyday processes:
- Incorrectly installed, faulty or poorly ventilated household appliances (typically boilers, cookers and fires) are common sources of carbon monoxide.
- Blockages in flues and chimneys can prevent carbon monoxide from escaping, instead forcing it back into households.
- Running fuel-powered appliances such as car-engines, generators and barbecues in enclosed spaces (eg. a garage) can result in a rapid build-up in carbon monoxide concentration. Blocked or faulty exhaust pipes can be similarly dangerous.
- Sudden power cuts to fuel-powered generators can cause improper combustion and monoxide poisoning. This was the case during the 2004 Hurricane disasters in Florida when 167 people forced to use generators were admitted to hospital with non-fatal poisoning.
- Paint fumes containing dichloromethane, which metabolises in the body to form carbon monoxide, can be an unexpected cause of monoxide poisoning.
Carbon Monoxide And Haemoglobin
Haemoglobin is a mammalian dioxygen-binding protein crucial for the process of respiration. The iron-containing heme unit, around which the protein chain wraps, binds to dioxygen molecules, allowing their transport by red blood cells throughout the bloodstream.
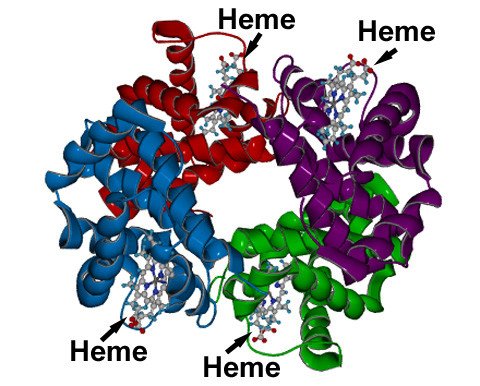
Carbon monoxide's toxicity arises due to its ability to bind to the heme unit's iron centre better than oxygen by donating a pair of electrons from its highest occupied molecular orbital, thereby greatly reducing the oxygen capacity of red blood cells. High-level exposure quickly displaces bound oxygen in the bloodstream and the victim soon suffocates.
Treatment Of Monoxide Poisoning
The treatment of severe carbon monoxide poisoning involves enclosure in a hyperbaric chamber and subjection to an atmosphere of near-pure dioxygen (as opposed to the 21% dioxygen content of Earth's atmosphere). This drastically reduces the half life - the time it takes for concentration to halve - of carbon monoxide in the body, from 300 minutes in normal air to 23 minutes in 100% dioxygen at three times atmospheric chamber pressure, so symptoms alleviate quickly.

Hyperbaric oxygen therapy https://goo.gl/zunPTJ
Carbon Monoxide Detectors
A device made to detect raised carbon monoxide concentrations must be sensitive and reliable - the lives of those living under it are at risk. There are three common types of carbon monoxide detectors:
- Biomimetic detectors simulate the process by which haemoglobin binds to molecules within the blood, and when monoxide concentration increases the gel within which this takes place changes colour. The colour change is detected by an independent sensor, which sounds an alarm.
- Metal oxide semiconductors comprise a circuit connected to a silica chip of variable electrical resistance. Contact with carbon monoxide lowers this resistance and increases circuit current. This increase is detected by an alarmed processor.
- Electrochemical detectors use a similar principle, although the circuit is attached to electrodes immersed in a chemical solution (rather than a silica chip). The current within the solution is altered by its carbon monoxide concentration, and this variation is detected by the circuit processor.
Chances are, one of these types of devices is alarming your home.
Always make sure your device is fitted with working batteries and regularly tested - it might just save your life!
If you enjoyed this article or are interested in all things science, follow my blog for daily doses of physics and chemistry, along with explanations for curious everyday phenomena.
References:
Chemistry 4th Edition by Housecroft and Constable
Carbon monoxide detectors
That's is a very nice post. I would avoid using tiny url if I were you. Using instead the following syntax:
[text-blablabla](the-full-url-without-spacings)I am always reluctant with clicking on links yielding to non verifiable places.
Thanks for the tip!
Beep beep. Hi @cjrc97!
You have used tip! in your comment - that`s my magic word for sending tips ;)
Click here if you wish to learn more!
Rofl!
How does a hyperbaric chamber cause the heme to break the bond with the carbon monoxide. If the electronegativity of the oxygen atom (carbon monoxide) is equal to the electronegativity of the oxygen atom (dioxygen), the carbon monoxide bond would not be broken to be replaced by the dioxygen.
The displacement of CO is less a mechanism and more a concentration-driven process. Hyperbaric dioxygen (in which an increased pressure means an increased number of molecules per unit volume) dissolves easily in the blood's plasma and competitively displaces CO molecules. This could be thought of as analogous to concentration, pressure and pH affecting the outcomes of a chemical reaction in a laboratory.
This is why the treatment does not immediately cure the patient; rather than a mechanism which occurs spontaneously within the plasma, the displacement is probability-based and occurs over time. For this reason HBOT is said to decrease the half-life of haemoglobin-bound CO in the blood and there is no definite time frame for complete CO removal.
Obviously the comprehensive answer to your question is more complicated, but I hope that helps.
Thank you for your response. It does make sense that the higher the concentration of dioxygen will result in more collisions. This would likely result in dislodging the bonded CO molecule and replacing it with a O2 molecule. I am aware that they increase the concentration of reactants to quicken the reaction time. Another technique is to introduce a catalyst but it this case, I don't think that would be an acceptable solution.