Let's Learn about Physical and Chemical changes (Chemistry Lesson)
Hello learners, I hope that I will be able to post continuously from now on. So this time it's one of my favorite section in Science, chemistry.

What is the difference between a Physical reaction and a Chemical reaction???

Carry out the following experiments which can be easily done at home
Experiment 01
Take 50ml of water to a beaker and add 1 tbs of salt and stir well.
Now heat the above solution till all the water evaporates.
What is your observation?
You will see that when you mix salt with water, the the physical properties of the salt changed. When you evaporate the water, you will see that salt has become to its original state.
Experiment 02
Take a magnesium strip and hold it over a flame and light it.
What are your observations?
You will see that the magnesium strip that you took before lighting was a bright and shining metal. Then it burnt with a bright flame. What was remained is some white color powder.
Now compare the two observations you got after doing experiment 01 and 02.
After the 1st experiment, salt became to it's original state. But after the 2nd experiment, the only thing that was remained was a white colored powder and the magnesium strip didn't came to it's original state.
We can call the 1st experiment a Physical change while we can call the 2nd a Chemical reaction.
Physical changes occur when matter undergoes a change that does not alter their chemical nature but may involve in a change in the physical properties.
A chemical reaction is a type of change in matter where one or more new substances are formed.
Accordingly in a Physical reaction, the substances can be brought back to their original state while in chemical reactions they can't be brought back to their original state but forms new substances.
Physical change Chemical change

What are the evidence to prove that a chemical reaction has occurred?
- Change in temperature
- Forming of gas bubbles
Experiment 03
Take some baking powder and Citric acid and mix them.
You will observe that the vessel becomes cool and forming of gas bubbles while the citric acid disappears.
- Forming of percipitate
- Change in color
Experiment 04
Take some water and add some Copper sulphate.
Add a piece of Zinc or Magnesium to it.
You will observe that a brown powder is deposited, the blue color reduces and the piece of Zinc or Magnesium gets smaller in size.
- Evolving gases
Experiment 05
Add some concentrated nitric acid to some Copper strips.
You will observe that the copper strips disappear and a brown color gas evolves.
The Law of Conservation of Mass
French scientist Antoie Lavoisier was the first person to introduce this.

What is the Law of Conservation of Mass?
The Law of Conservation of Mass states that the total mass remains unchanged after a chemical reaction that takes place in a closed system.
Let's do this experiment for further clarifications.
Experiment 06
Place few matchsticks in a boiling tube and cover the mouth of the boiling tube with a balloon.
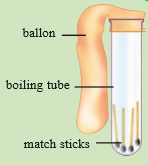
Measure the mass of it.
Now hold it to a fire until the matchsticks get burnt.
After it is cooled, measure the value.
What can you observe?
When you measure the mass of the setup before the matchsticks are burnt and after the matchsticks are burnt, the mass is not changed. This clarifies the Law of Conservation of Mass.
Why a ''Closed system???''
Do the above Experiment 06 without closing it's mouth by a balloon.
Then you will observe that the mass has decreased.
So when we do it in a closed system, nothing can enter nor go out of the setup. So the mass stays unchanged.
So we can conclude that the mass stays unchanged in a chemical change that takes place in a closed system.
_________________________________________________________________________________
That's all for today
All these information is from my Science text book
Thank you for stopping by
Hope you learnt something
UPVOTE-FOLLOW-COMMENT-RESTEEM
Thank you
@driva has voted on behalf of @minnowpond.
If you would like to recieve upvotes from minnowponds team on all your posts, simply FOLLOW @minnowpond.
Thank you