How to Simulate Corrosion of Stainless Steel in Seawater
Summary
This article show the corrosion behaviour of Stainless steel in artificial seawater, and under mechanical load such as stress in the surface of material. The C-ring specimens are chosen following to ASTM G-38 standard to resemble the stress conditions during the applied load. The specimens are immersed in 3.5% wt of Natrium Chloride (NaCl) solution as artificial of seawater and the variable conditions at a fixed stress of 379MPA and immersed for 720 hours.
How To Do It
The material were formed into C-Ring shapes following to the ASTM G-38 Standard (as shown in Figure 1) and were used in the experimental setup. In which ODf is the outside diameter after stressing, f is the desired stress, delta is the deflection of the OD given by the desired stress, D is the mean diameter equal to OD – thickness. The surface model was selected based on actual problems that that occured on any real problems such as aircraft wings, helicopter longerons, and also petrochemical storage tanks.
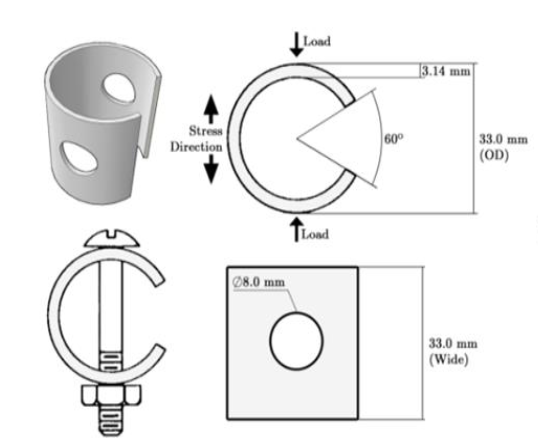
Figure-1:Dimension of specimen following to the ASTM standard
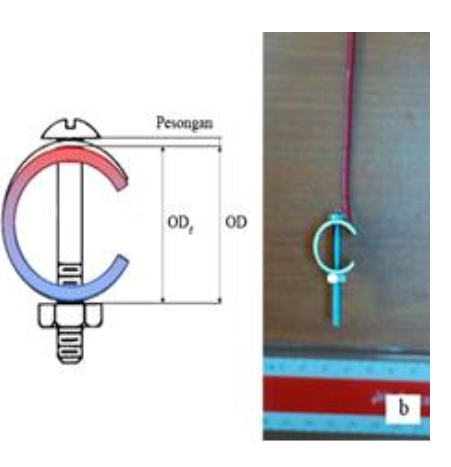
Figure-2: Illustration and Photograph of real specimen
Three immersion times were used to evaluate in polarization behaviur. For this purpose, I used the ASTM G44 standard as a guide in making artificial seawater. 3.5 % NaCl (Sodium Chloride) was added to the required quantity of distilled water. Figure 3 shows the exposed area on the specimen and the concentration of the specimens in the 3.5% NaCl solution.
Furthermore, in order to obtain an appropriate field of vulnerability on the specimen, a protective coating process was carried out using isolators. The exposed part of the specimen was only opened as wide as 10 mm in the area estimated to receive the highest levels of stress.
The final stage of the specially made salt bridge was aimed straight at the exposed area on the specimen. Figure 3 shows the exposed area on the specimen and the immersion of the specimens into the 3.5% NaCl solution.
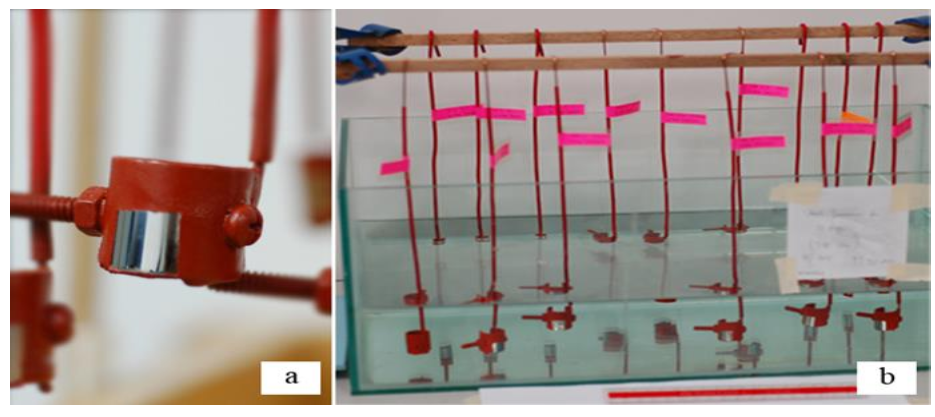 Figure-3: Exposed area of specimen (a) and the immersion process(b)
Figure-3: Exposed area of specimen (a) and the immersion process(b)Tools
A portable model of the Potentiostat type ZIVE PP1 (Korea) connected to an electrochemical cell was used for measurements. The C-ring specimen was the working electrode (WE). A Silver Chloride Electrode (Ag/AgCl) was used as the Reference Electrode (RE) and a graphite bar was prepared as the Counter Electrode (CE).
Results
Using the potentiodynamic polarisation measurement method and microscopic observation, the corrosion polarisation behaviour of austenitic stainless steel became more active in cases when immersion and stress are applied simultaneously. The stress on the surface of the specimen shows a significant effect on the polarisation mechanism in the analysed area. The result also shows that the locations of pitting corrosion are apparent on the highest stress area. There is useful information to analyse the corrosion behaviour especially for applications under mechanical loading in corrosive environments, it drives the corrosion behaviour to the critical condition.
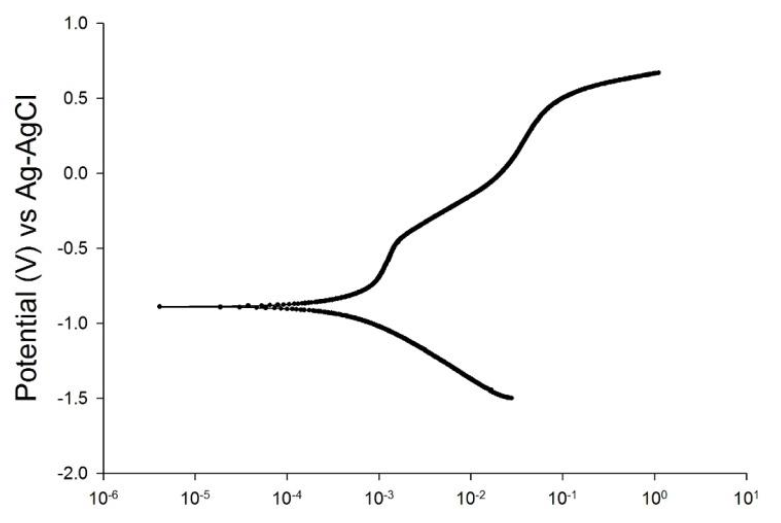 Figure-5: Corrosion Polarization Curve of Stainless steel during under mechanical load.
Figure-5: Corrosion Polarization Curve of Stainless steel during under mechanical load.Many hypotheses of the corrosion damage on the stainless steel in sodium chloride have been confirmed, particularly for 304 austenitic stainless steel. The elastic zone stress under the yield strength of this material contributed to the pitting corrosion in cases when immersion and stress are applied simultaneously. The essential factor for decreasing the corrosion behaviour of 304 stainless steel is the level of stress during the applied load. The immersion time is not strongly affected by the corrosion behaviour of non-stressed material. The pitting corrosion triggered by stress occurred on the critical surface of austenitic stainless steel. Corrosive environments, such as seawater or sodium chloride solutions, drove the corrosion behaviour to a critical condition. The location of stress influenced the mechanism of the corrosion polarisation. The 94 MPY of the corrosion rate shows that the effect of stress could be critical if austenitic stainless steel is found in a corrosive environment.
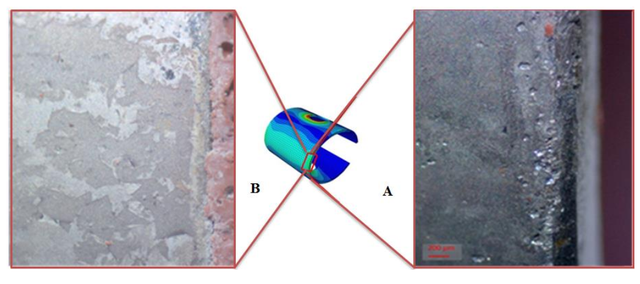 Figure-6: Pitting corrosion distribution on the edge of the C-Ring surface(a), and the edge of the C-Ring surface under less stress.(b)
Figure-6: Pitting corrosion distribution on the edge of the C-Ring surface(a), and the edge of the C-Ring surface under less stress.(b)Disclaimer:
I published this article as work paper as title “Stress corrosion damage on austenitic stainless steel in sodium chloride” and accepted in IJAME with this DOI address!

@caknuris
I encourage everybody to use their own texts, and images as much as possible.
stay be yourself, this is a better way to build steemit.com