The first law of thermodynamics - ILLUSTRATION
The first law of thermodynamics
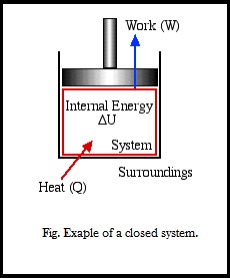
The first law of thermodynamics or also known as law of conservation of energy states that "The total amount of energy in any isolated system remains constant, and cannot be created or destroyed, although it may change forms." which alternatively means energy transformation is accomplished through energy transfer as work and/or heat. Work and heat are the forms that energy can take in order to be transferred across the system boundary.
energy Entered − Energy left = change of energy within the system
Q - W = ΔU {In kJ}
Where:
Q is the Heat transferred to the system
W is work done by the system
ΔU is the change in internal energy.
Heat (Q)
Energy transferred between the boundary of a system in form of heat always results from the difference in temperature between the system and its surroundings. We will not consider the mode of heat transfer, whether by conduction, convection or radiation, thus the quantity of heat transferred during any process will either be specified or evaluated as the unknown of the energy equation. By convention, positive heat is that transferred from the surroundings to the system, resulting in an increase in internal energy of the system.
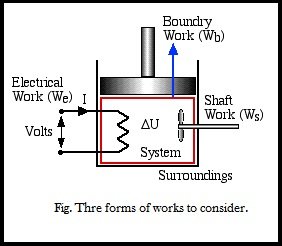
Work (W)
There are three modes of work transfer across the boundary of a system,
considered as below:
1. Boundary work Wb {Piston Cylinder}
2. Shaft work Ws {Paddle Wheel}
3. Electrical work We {Volts.I(Amps).time}
Right now are mainly dealing with Boundary Work due to compression or expansion of a system in a piston-cylinder device as shown above. In all cases we assume a perfect seal (no mass flow in or out), no friction loss, and quasi-equilibrium processes in that for each incremental movement of the piston equilibrium conditions are maintained. By convention positive work that is done by the system on the surroundings, and negative work is that done by the surroundings on the system, Thus since negative work results in an increase in internal energy of the system, this explains the negative sign in the above energy equation.
Boundary work can be evaluated by integrating the force F multiplied by the incremental distance moved dx between an initial state to a final state. We normally deal with a piston-cylinder device, thus the force can be replaced by the piston area A multiplied by the pressure P, allowing us to replace A.dx by the change in volume dV, as below:
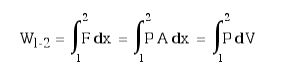
This is also shown in the schematic diagram below the integration can be represented by the area under the curve.
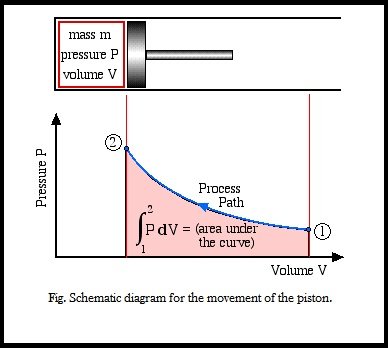
The Work-done is a path Function, not a property, thus it is dependent on the process path between the initial and final states.
typical process paths of interest (As below):
- Isothermal (constant temperature process)
- Isochoric or Isometric (constant volume process)
- Isobaric (constant pressure process)
- Adiabatic (no heat flow to or from the system during the process)
As described equation can also be written in the terms of specific work-done.
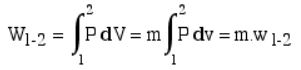
Where P is Pressure {kPa},
V is volume {m³}, m is Mass {kg},
v is specific volume {m³/kg}, W is work done {kJ}
w is specific work done {kJ/kg}
With the equation above we can state that work done by the system on the surroundings is positive and work done by the surroundings on the system is negative. And we can see the example of the first law all around us from IC Engines to Water turbines.
Images & References: Wikipedia.com , An article on ohio.edu
I would also recommend this short video by curiosityhub on Youtube.

Daily Fun Fact #14 --
"Give a man a parachute and he will fly for a day.
but give the man a faulty parachute, & he will fly for the rest of his life. "
OH dang bro, it seems you got cheeta. Put all the stuff you got from the webs in quotes. Or change the sentences
I didn't get the cheetah, the cheetah got me. lol. thanks for the tip, will keep that in mind. :D