Evaluating the reactivity of metals: Prediction of redox reactions with metallic species and cations.

Metals represent an important group on the periodic table with various functions. An example of this is our organism, where the presence of certain metals such as iron, zinc, magnesium, etc. is vital. (always in an appropriate proportion). In the industrial sector, metals play a fundamental role, whether in a factory, construction company, etc. The beauty of these elements has been instinctively appreciated by humans since ancient times, which is why gold has historically acquired great value, as its shine never disappears and it is very resistant to oxidation. To understand thermodynamically the oxidation-reduction process between metals and metal cations, it is necessary to understand the series of activity of metals, based on this, different experiments were carried out to experimentally observe the reducing power of metals such as copper, zinc, lead, iron, tin, aluminum and magnesium in the presence of salts that provided metal cations when dissociated. In addition, the influence of temperature on the oxidation of certain metals was observed, also considering bubbling and pH change for thorough analysis.
Theoretical Basis
Some reactions are called oxide-reduction reactions or redox reactions, as they represent processes in which one or more electrons are transferred between the reagents, causing a change in their oxidation states.
In order to correctly identify an oxidation-reduction reaction, we need to know the transfer process of the electrons gained by the substance being reduced and those lost by the substance being oxidized. The concept of oxidation numbers (also called oxidation states) was designed precisely to define and identify the processes that occurred in the reactions. The oxidation number of an atom in a substance is the actual charge of the atom when it is a mono-atomic ion; in other cases, it is the hypothetical charge assigned to the atom based on a set of rules.
Corrosion of iron and other metals, such as corrosion on the terminals of a car battery, is a process we all know. What we call corrosion is the conversion of a metal into a metal compound by a reaction between the metal and a substance in its environment. The production of rust involves the reaction of oxygen with iron in the presence of water.
Reactions can also be classified as displacement reactions, in which an ion or atom of a compound is replaced by an ion or atom of another compound, in this case an oxidized one, for example:
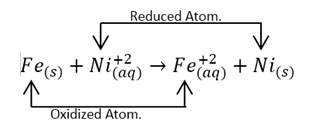
It is evident that iron has replaced nickel in the nitrate molecule.
The species that oxidize, favor the reduction of another species, so they are called reducing agents to the species that oxidize. Similarly, when a species is reduced, it would be favoring the oxidation of another compound, being called reducing agents. Example:
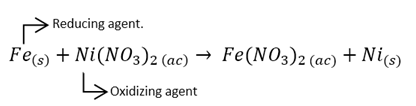
Oxidation is the loss of electrons in a compound, while reduction is the gain of the electrons.
It is possible to predict when a metal can oxidize if it is found in aqueous, acidic or saline solutions (in this case it will be tested with metallic salts) considering the electronegativity of each of them simultaneously with their ability to attract electrons (electronic affinity).
The standard potential of a cell, Eo, which corresponds to each metal that can be oxidized or reduced, is the potential originated when all species are present in the standard thermodynamic conditions, i.e. concentrations of 1M for solutes in solution and 1atm for gases, and 25º C, in a hydrogen electrode (which has a potential of 0.00). These values can then be tabulated and the reducing power (ability to reduce to another species) of each element can be observed, this table is called the metal activity series.
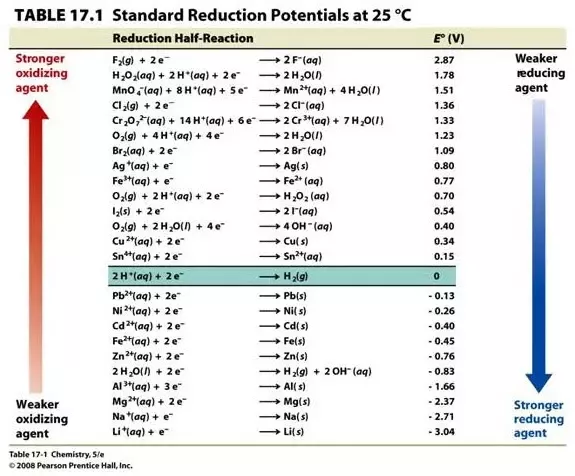
Following theoretically the series of activity of metals, it can be said that each metal that is above another can be displaced (reduced) by some compound, such as salt. The reducing force (ease of oxidation) increases as you move up the table.
Below are some laboratory experiments with which you can check the above as part of the theory of this interesting topic.
Reactivity between metals and metal cations.
Procedure:
To carry out this experience, 4 test tubes labeled with the symbols of the 4 metals to be studied (Cu, Zn, Pb, Fe) were taken, each one with a small piece of the corresponding metal and 2 ml of copper (II) nitrate solution (Cu(NO3)2) 0.05 M added to it. Then the changes that might or might not occur were observed for 10 minutes.
This Procedure was repeated using silver nitrate (AgNO3), zinc nitrate (II) (Zn(NO3)2), ferric nitrate (Fe(NO3)3), mercury nitrate (Hg(NO3)2) and lead nitrate (II) (Pb(NO3)2); all with a concentration of 0.05 M.
Results:
In order to fully understand the range of activity of metals, it is necessary to subject them to solutions containing a certain concentration of some cation of a metallic nature. In this case, salts (nitrates) were used to verify this effect.
The metals used were found in small pieces, in some cases as iron in addition to being small were of little thickness. The copper was arranged in thin sheets.
It is important to note that the metals used were not 100% pure, as they contained traces of other compounds, this could affect some of the observations or results of each of the experiences, however, the level of purity is sufficient for most of them.
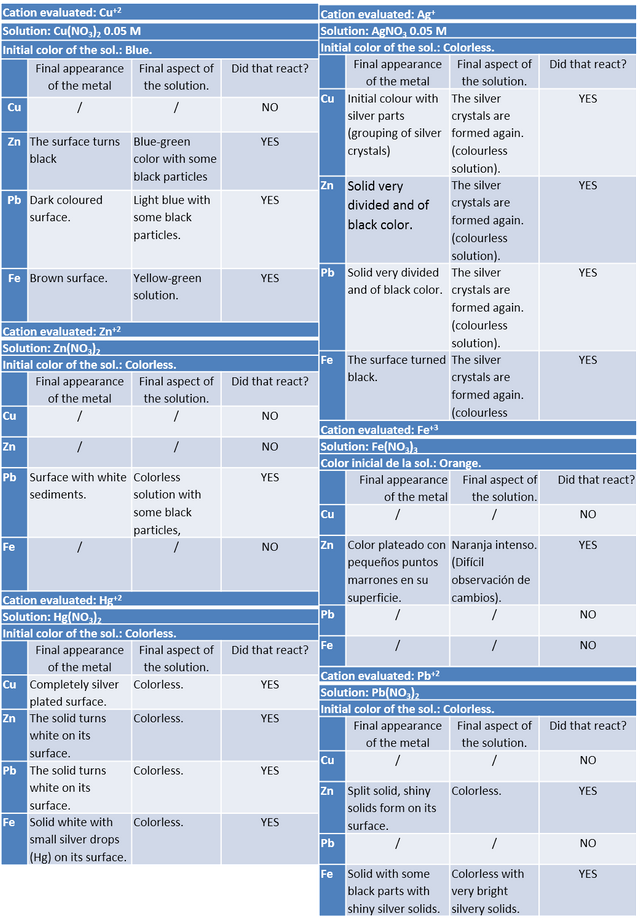
According to the results obtained and using the series of activity of the metals, each of the experiments carried out can be verified.
Copper and iron could not displace the Zn+2 cation of the nitrate molecule as its reducing power was not strong enough; thermodynamically this could be calculated with the standard reduction potentials of each metal, for example:
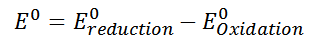
If zinc is taken hypothetically as a reduced species, and copper as an oxidized species, the formula is replaced with the potentials corresponding to each element as follows:
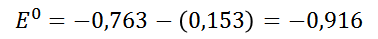
The result obtained is negative, which represents a positive Gibbs free energy, representing ando this thermodynamically a non-spontaneous process, which is interpreted as a process that cannot occur in the given conditions (standard conditions).
Similarly, this happens with the Fe+3 cation which could not be reduced by copper (Cu) and lead (Pb); the Pb+2 cation which could not be reduced by copper (Cu).
In most cases there was a relatively fast reaction (no more than 15 minutes), and in the case of copper nitrate solution there was a change in colour in the oxidised metal species, which turned dark. As for its solution, when Zn was added, the solution turned green, this being a decrease of the intense blue color that the solution originally presented.
Silver nitrate was able to finely divide zinc and lead, forming in all cases a precipitate similar to cotton, grey in colour, which was a grouping of the newly formed silver crystals.
The ferric nitrate only reacted with zinc, while the mercury nitrate and lead (II) nitrate when they reacted, generated white solids (some very bright and in the case of mercury, some small silver drops that were fixed on the surface of the metal with which it reacted.
Reactivity between metals and an acid.
Procedure:
In this experience, 7 test tubes were labeled with the symbols corresponding to the small piece of metal inside (zinc, tin, aluminum, copper, iron, lead, and magnesium).
5ml of 6M hydrochloric acid (HCl) was added to each of them. Every possible change that might occur was carefully observed for 5 minutes.
Those tubes that did not present any change, were introduced in a water bath which was heated until boiling, it was observed which of these first reacted.
Results:
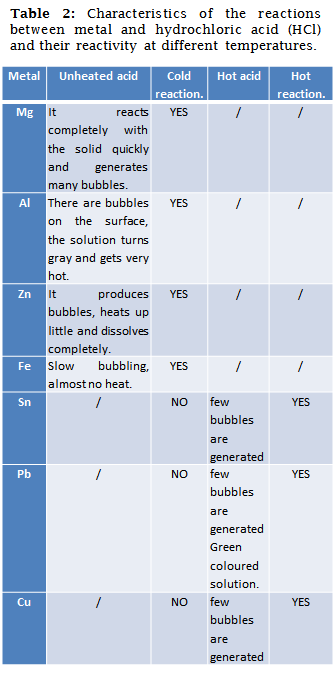
Some metals are capable of displacing hydrogen from hydrochloric acid (HCl), as their reducing power allows it. Thermodynamically many of these reactions are favored, however some of them require a change of temperature to occur. This is why some of the samples had to be heated, thus favouring the reaction.
A bubbling product of the gaseous hydrogen released in each of the reactions was always obtained, which can be generally expressed as:
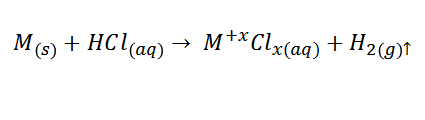
M being the metal studied and X being its valence after oxidation.
In table 3 the metals can be seen in order according to their reactivity to acid, magnesium, aluminum and zinc reacted violently producing a large number of bubbles and a notable increase in heat. The iron turned out to be a little slower than the previous ones and the temperature change was not very noticeable.
After observing that tin, lead and copper did not react at room temperature, they were heated to favor this reaction, of them the metal that reacted first was tin, followed by lead and finally copper. These results are theoretically based on the activity series of metals, since the least likely to react with acid based on its reduction potential is copper.
Once again, each of the tests carried out on the different metals satisfied the theoretical propositions regarding their reactivity with acid.
3. Reactivity between a metal and water.
Procedure:
To check the reactivity of a metal with water, a test tube was blunted where a small piece of magnesium was placed. The pH of the distilled water to be used with the help of the indicator paper was measured and 5 ml of it was added to the test tube.
The test tube was heated in a water bath to show signs of reaction (bubbling). After he started to react he was left 5 more minutes in the water bath.
At the end, the pH of the solution contained in the tube was measured again using the indicator paper and compared with the initial pH of the distilled water.
Results:
The most reactive metals, which have a great reducing power, are capable of displacing hydrogen from the water molecule. In this case, the reactivity of magnesium in water and its influence on its pH were studied.
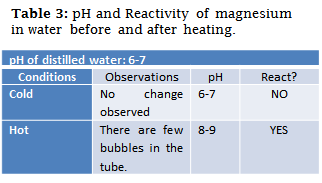
It was noted that the reaction did not occur at room temperature, initially the pH value of the distilled water was between 6-7 on the paper frame indicator.
After the test tube was heated in a water bath, few bubbles were observed to ensure that the reaction was occurring, after waiting long enough, the pH was measured to quantify its variation, the pH was found to be a value between 8-9, which shows an increase in the same product of the next reaction:
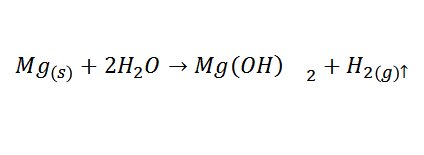
When magnesium hydroxide is formed, it will react with water, providing oxidyl (OH-) ions to the solution, which causes an increase in the pH of the solution (it becomes a basic solution).
It is consistent with the result obtained by measuring the water with the indicator paper, thanks to the heating of the water, which thermodynamically favoured the reaction with the magnesium.
General Conclusion
Some results were much more evident than others, since their physicochemical properties made the reaction slow and not very visible. A specific case to be highlighted could be zinc (Zn) which, being in the presence of Fe(NO3)3 showed very few signs of oxidation, theoretically zinc could easily reduce iron, however to be able to observe this phenomenon it was necessary to look at the solid very closely, thus showing some small brown spots resulting from the expected reaction. This type of consideration was presented as some of the metals were not pure enough and contained many traces of other compounds, however, the reaction was observed and the result was satisfactory.
In the case of pH measurement, the indicator paper used had a scale that allowed the pH to be measured every two units, which could be inaccurate for the practice performed, although the change in pH was sufficiently visible.
In general, it can be concluded that the results obtained were really satisfactory with the theoretical guidelines exposed corresponding to the reactivity of metals in the presence of metal cations, non-oxidizing acids and water.

I hope this article has been to your liking, I think it is important and interesting, as this type of oxidation process can be observed daily in infrastructure, ships, doors, etc..
We are still waiting for the great developments that the future will bring, you decide whether to be a spectator or a doer. Every day we can learn something new.
Thank you for reading.
References:
All the images of my authorship were edited and processed using the software PowerPoint 2016.
- Theodore Brown, Eugene LeMay, Bruce Bursten, Julia Burdge, (2004), Chemistry. The central science. (9th Edition). Pearson Education, S.A.
Douglas Skoog, Donald West and James Holler (2006) Fundamentals of Chemistry Analytical (4th Edition) Editorial Reverté.
CRC HandBook of Chemistry and Physics, 86th Edition, CRC Press 2005

Posted from my blog with SteemPress : http://aleestra.vornix.blog/evaluating-the-reactivity-of-metals-prediction-of-redox-reactions-with-metallic-species-and-cations/
